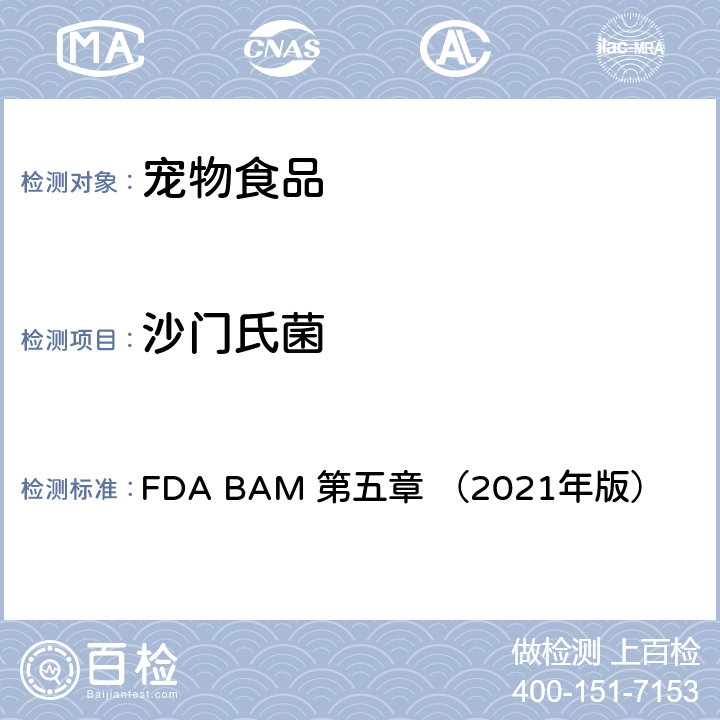
FY 2021 MDUFA User Fees
美国FDA医疗器械类2021注册年费
The Fees for Fiscal Year 2021 (October 1, 2020 through September 30, 2021) are as follows:
FDA发布了医疗器械2021财年收费案(适用于2020.10.01-2021.09.30适用)
Application Type | Standard Fee | Small Business Fee |
510(k) | $12,432 | $3,108 |
513(g) | $4,936 | $2,468 |
PMA, PDP, PMR, BLA | $365,657 | $91,414 |
De Novo Classification Request | $109,697 | $27,424 |
Panel-track Supplement | $274,243 | $68,561 |
180-Day Supplement | $54,849 | $13,712 |
Real-Time Supplement | $25,596 | $6,399 |
BLA Efficacy Supplement | $365,657 | $91,414 |
30-Day Notice | $5,851 | $2,926 |
Annual Fee for Periodic | $12,798 | $3,200 |
说明:所有类型的510(k)(传统的、缩写的和特殊的)都需要支付年费。然而,对于代表FDA认可的第三方审查员
提交给FDA的510(k)份报告,不收取年费。
小型企业只要有一个经批准的SBD,总收益或销售额在3000万美元或以下,就有资格在他们的**个
PMA、PDP、PMR或BLA上免除费用。
注册机构年费:$5,546。
对于小型机构、企业或团体,没有豁免或削减——所有机构必须支付设立注册费。